Introduction: Graft versus host disease (GvHD) is a complex immune process that can affect up to 50% of patients undergoing allogeneic stem cell transplantation (allo-SCT). Even though prophylactic immunosuppressive regimens have been established over the last decades, GvHD is still one of the main causes of morbidity and mortality among these patients. Monocytes are cells of the innate immune system, that represent, as professional antigen-presenting cells, a connection to the adaptive immune system, and play a role in several autoimmune disorders. They are classified according to the expression of CD14 and CD16 into classical (CD14++/CD16-), intermediate (CD14++/CD16+), and non-classical (CD14+/CD16++) monocytes. Previous research has shown higher levels of intermediate monocytes in pediatric patients with GvHD. Furthermore, non-classical monocytes in allogenic bone marrow grafts have also been correlated with the development of GvHD.
The expression of other surface markers such as HLA-DR and CD300e (IREM-2) are related to monocyte function and engagement in inflammatory processes. Nevertheless, the role of CD300e in GvHD remains unclear. Here, we present a description of monocyte subgroups and their expression of HLA-DR and CD300e in allo-SCT patients with or without GvHD as well as in healthy subjects.
Methods: Transversal observational study with patients undergoing allo-SCT. Peripheral blood of patients was collected once after engraftment. Both acute and chronic GvHD patients were enrolled. Five healthy volunteers served as controls. Flow cytometry was performed to determine the immunophenotype of monocytes according to their expression of CD14 and CD16 stratifying into classical, intermediate, and non-classical subgroups as described above. In addition, the expression of HLA-DR and CD300e was analyzed for all monocytes and individual subgroups. Clinical characteristics are described in the table below. Statistical analysis was performed using GraphPad Prism 9. Comparisons between groups were made using non-parametrical tests and tests for multiple comparisons (Mann-Whitney and Kruskal-Wallis respectively).
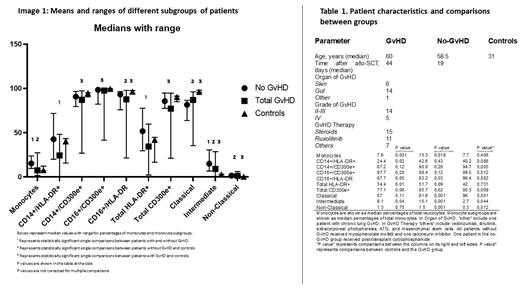
Results: 29 patients were enrolled. Two patients with GvHD were excluded due to neutropenia. 19 patients presented GvHD grade II or higher. Of those, ten had acute GvHD, five had prolonged acute GvHD, two had late onset acute GvHD, and two had chronic GvHD. Eight patients did not show GvHD or had only mild skin disease (grade one). The mean age was 60 years for all patients, 62.5 and 58.5 years among GvHD and non-GvDH patients, respectively. The image summarizes patient characteristics and comparisons.
We compared transplant recipients with and without GvHD which each other and with healthy controls. In single comparisons, patients with GvHD showed significantly lower percentages of total monocytes as well as CD14+/HLA-DR+, total HLA-DR+, and intermediate monocytes compared to patients without GvHD. In addition, we observed a trend toward lower levels of CD16+/HLA-DR+ and CD300e-expressing monocytes in GvHD patients.
When compared to healthy controls, allogeneic recipients without GvHD showed significantly higher levels of total monocytes, intermediate monocytes, and CD16+/HLA-DR+ monocytes and lower levels of classical and non-classical monocytes. Compared to controls, patients with GvHD showed significantly lower levels of total monocytes expressing CD300e (also true in all subgroups) as well as lower levels of classical monocytes, but higher levels of intermediate and non-classical monocytes. P values are shown in Table 1. Means and ranges for all groups are represented in Image 1.
Conclusions: In our cohort, patients with GvHD showed higher levels of intermediate and non-classical monocytes compared to controls, however, these populations were lower when compared to allogeneic recipients without GvHD. This could indicate a consumption process similar to that observed in COVID-19 patients and a direct involvement of these monocytes in the GvHD process. In addition, we report for the first time the involvement of CD300e in GvHD, which was significantly lower expressed in the GvHD patients, compared to controls. However, there was only a strong trend compared to transplanted non-GvHD patients. This indicates a possible contribution of CD300e in the pathogenesis of GvHD. Further studies are needed in order to confirm these findings.
Disclosures
Age Kos:Pfizer: Other: Travel funds. Bittenbring:AstraZeneca: Honoraria. Lesan:Pierre Fabre: Other: Travel and Congress Grant. Christofyllakis:AstaZeneca: Other: Educational/travel grant, Research Funding; Roche: Honoraria; Novartis: Consultancy, Other: Educational/travel grant; Takeda: Consultancy; Hexal: Other: Educational/travel grant; Celgene: Other: Educational/travel grant; Jazz Pharmaceuticals: Other: Educational/travel grant; Abbvie: Other: Educational/travel grant. Bewarder:Janssen Pharmaceuticals: Consultancy, Honoraria; AstraZeneca: Consultancy; Incyte: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal